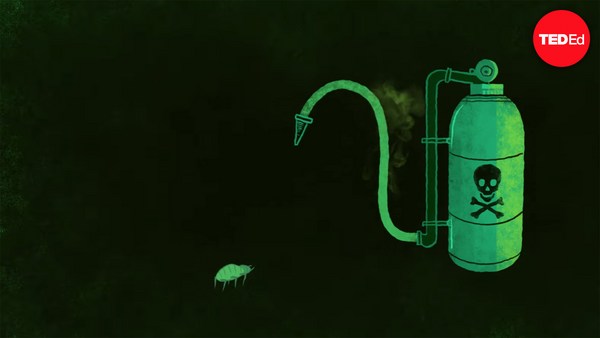
From 1952 to 1953, Sydney detectives investigated a staggering number of murder and attempted murder cases that were unrelated yet shared a common element: thallium poisoning. The secret to thallium toxicity lies in its structural similarity to potassium— an element that helps regulate the body's fluids, initiate muscle contraction, and transmit nerve signals. If even a small amount of thallium sneaks its way into the body— for example, through a tainted tea or a slice of cake— it easily supplants potassium, causing the body to slowly and painfully shut down. At the time, thallium's risks were well known, so how were the perpetrators able to get their hands on such a lethal element?
And thallium isn't the only dangerous element on the periodic table. Within this tabular array loom several potential threats, each with their own unique method of imposing destruction.
Some elements, like thallium, are dangerous due to their toxicity. Once they enter the body, they wreak havoc on the biological systems that keep us alive. Lead, for example, switches places with the body's essential metals like calcium, in turn disrupting neuronal communication in the brain. Traveling through the bloodstream, it also generates toxic levels of molecules known as reactive oxygen species, which over time can stress and kill cells. Mercury's toxicity was made famous in the 19th century due to its widespread use in felt hat production. Prolonged exposure made hat makers ill with what was later known as "Mad Hatter" disease, with symptoms that included personality changes, emotional disturbances, and tremors. Mercury is quick to react with certain parts of proteins found throughout the body. And upon binding, mercury twists the proteins into different shapes, rendering them useless.
Some elements are dangerous because of how they respond, react, or even explode in the outside environment. Top reactive elements reside in the first column of the periodic table and are known as alkali metals. They're rarely found in their pure elemental form, as alkalis readily donate the single electron in their outer shell to whatever's around to form more stable ionic compounds. This can lead to violent results— pure cesium, for example, bursts into flames when exposed to air, and explodes when dropped in water. Francium is likely the most reactive alkali based on its position in the periodic table, but we don't know for sure. With a half-life of 22 minutes at most, it's thought that less than an ounce exists on Earth at any one time.
But perhaps the most threatening elements are those that silently emit. Known as radioactive elements, the substances readily release energy, or decay, due to their highly unstable nuclear composition. This reactive nature is what's harnessed to create some of the world's most dangerous nuclear weapons. Radioactive elements typically emit energy in the form of alpha particles, beta particles, neutrons, or electromagnetic radiation. While all dangerous, alpha particles, which consist of two neutrons and protons bound tightly together, can be particularly hazardous. Heavy and positively charged, if alpha particles find their way into the body, they can easily bombard and kill any cell in their path. In fact, it's theorized that a single gram of one alpha emitter, polonium, could kill upwards of 50 million people. Polonium was first discovered by Marie Curie, and tragically her daughter, researcher Irene Joliot-Curie, may have been one of its first victims after she was exposed in a lab accident. Polonium is rare in nature with few commercial uses, so only a small amount is synthesized each year.
Thallium, on the other hand, wasn't so difficult to find in the early 1950s in Austalia. At the time, Sydney was plagued with chronic rat infestations. And thallium was the main ingredient in the popular and cheap rat poison called Thall-Rat. Thankfully, detectives were able to connect the dots, and in 1953 Australian Parliament effectively banned all sale of thallium.