From the smallest single-celled organism to the largest creatures on earth, every living thing is defined by its genes. The DNA contained in our genes acts like an instruction manual for our cells. Four building blocks called bases are strung together in precise sequences, which tell the cell how to behave and form the basis for our every trait. But with recent advancements in gene editing tools, scientists can change an organism’s fundamental features in record time. They can engineer drought-resistant crops and create apples that don’t brown. They might even prevent the spread of infectious outbreaks and develop cures for genetic diseases. CRISPR is the fastest, easiest, and cheapest of the gene editing tools responsible for this new wave of science. But where did this medical marvel come from? How does it work? And what can it do?
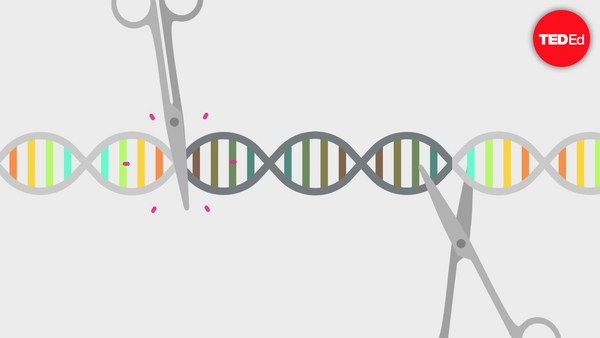
Surprisingly, CRISPR is actually a natural process that’s long functioned as a bacterial immune system. Originally found defending single-celled bacteria and archaea against invading viruses, naturally occurring CRISPR uses two main components. The first are short snippets of repetitive DNA sequences called “clustered regularly interspaced short palindromic repeats,” or simply, CRISPRs. The second are Cas, or “CRISPR-associated” proteins which chop up DNA like molecular scissors. When a virus invades a bacterium, Cas proteins cut out a segment of the viral DNA to stitch into the bacterium’s CRISPR region, capturing a chemical snapshot of the infection. Those viral codes are then copied into short pieces of RNA. This molecule plays many roles in our cells, but in the case of CRISPR, RNA binds to a special protein called Cas9. The resulting complexes act like scouts, latching onto free-floating genetic material and searching for a match to the virus. If the virus invades again, the scout complex recognizes it immediately, and Cas9 swiftly destroys the viral DNA.
Lots of bacteria have this type of defense mechanism. But in 2012, scientists figured out how to hijack CRISPR to target not just viral DNA, but any DNA in almost any organism. With the right tools, this viral immune system becomes a precise gene-editing tool, which can alter DNA and change specific genes almost as easily as fixing a typo.
Here’s how it works in the lab: scientists design a “guide” RNA to match the gene they want to edit, and attach it to Cas9. Like the viral RNA in the CRISPR immune system, the guide RNA directs Cas9 to the target gene, and the protein’s molecular scissors snip the DNA. This is the key to CRISPR’s power: just by injecting Cas9 bound to a short piece of custom guide RNA scientists can edit practically any gene in the genome.
Once the DNA is cut, the cell will try to repair it. Typically, proteins called nucleases trim the broken ends and join them back together. But this type of repair process, called nonhomologous end joining, is prone to mistakes and can lead to extra or missing bases. The resulting gene is often unusable and turned off. However, if scientists add a separate sequence of template DNA to their CRISPR cocktail, cellular proteins can perform a different DNA repair process, called homology directed repair. This template DNA is used as a blueprint to guide the rebuilding process, repairing a defective gene or even inserting a completely new one.
The ability to fix DNA errors means that CRISPR could potentially create new treatments for diseases linked to specific genetic errors, like cystic fibrosis or sickle cell anemia. And since it’s not limited to humans, the applications are almost endless. CRISPR could create plants that yield larger fruit, mosquitoes that can’t transmit malaria, or even reprogram drug-resistant cancer cells. It’s also a powerful tool for studying the genome, allowing scientists to watch what happens when genes are turned off or changed within an organism.
CRISPR isn’t perfect yet. It doesn’t always make just the intended changes, and since it’s difficult to predict the long-term implications of a CRISPR edit, this technology raises big ethical questions. It’s up to us to decide the best course forward as CRISPR leaves single-celled organisms behind and heads into labs, farms, hospitals, and organisms around the world.